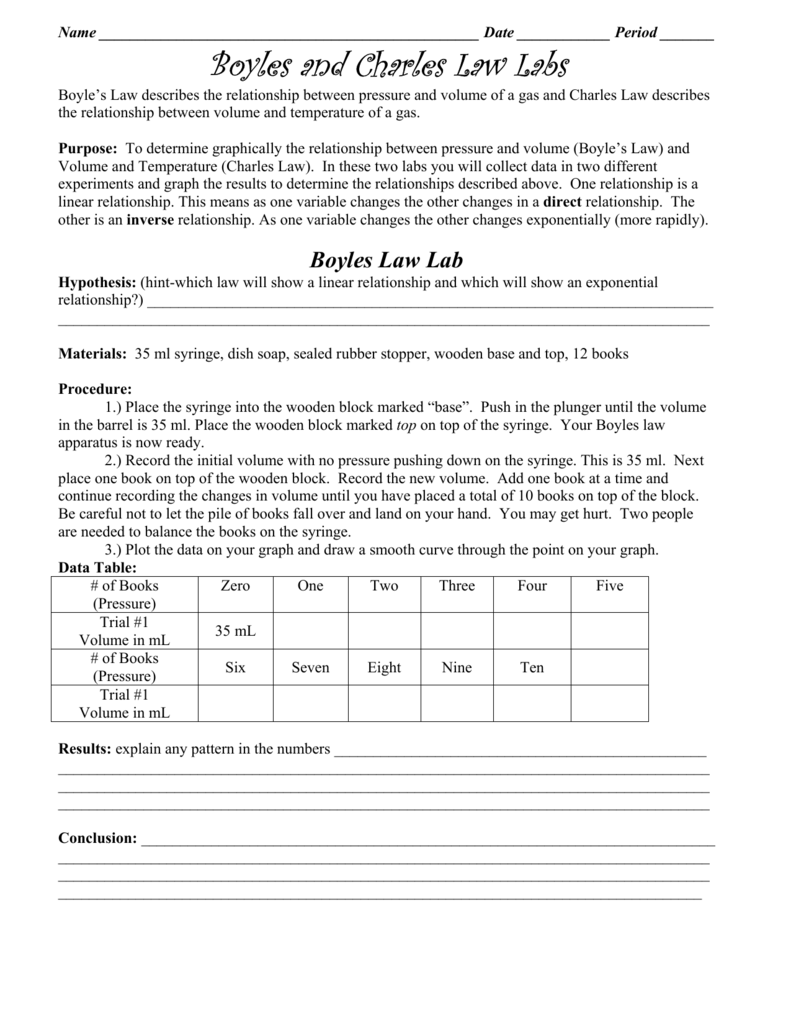
PVkconstant The constant kk will depend on the number of moles and the temperature. 2 In a thermonuclear device the pressure of 0050 liters of gas within the bomb casing reaches 40 x.
The Boyles Law is one of the main Laws that are used to study the behavior of gases.

. All Chemistry Classes Boyles Law Lab Name _____ Purpose. SHS Work Immersion Portfolio. Boyles law states that the pressure of a fixedshow more content Its mathematical expression is V_1T_1 V_2T_2.
Ohms law - Lab report. Robert Boyle discovered the inverse relationship between pressure and volume in 1662. Boyles Law Lab Report 1.
Connect the open end of the syringe to the pressure sensor. The law states that an increase in pressure would lead to a decrease in the volume of a confined gas. General Chemist ry 1.
The experiment clearly demonstrated this phenomenon and through the experiment the Boyles constant was found to be around 6400236pamL. This is important because the more pressure an object has the less volume it has. This law of chemistry is one of the gas laws of chemistry.
Nine Bottl es. Besides that the Avogadros law state that the volume of a mixed amount of gas is directly proportional to the number of gas molecules at the constant temperature and pressure where its equation is V_1n_1 V_2n_2. Pressure-Volume Relationship in Gases Boyles Law INTRODUCTION The primary objective of this experiment is to determine the relationship between the pressure and volume of a confined gas.
What is the new pressure of the gas. 2 pts It is evident that the student was able to connect the learning goals of the experiment with data obtained in the experiment. Any units will work here.
Ankon Rahman Introduction Boyles law is an established law of chemistry given by Robert Boyle 1627-1691. Boyles law can be define P α 1V or PVk where P is the pressure of the gas V is the volume of the gas and k is the constant. 2 pts The sentences are comprehendible to the reader.
Synthesis and Analysis of Sodium Hydrogen Carbonate and Sodium Carbonate. Students will then complete a formal lab report. EXPERIMENTAL PROOF OF BOYLES LAW.
So in this experiment we have measure the pressure of the gas in the pressure cylinder whether the pressure is increase or decrease when the volume of the gas being decrease. P is the pressure V is the volume and K is the constant. 1 100 L of a gas at standard temperature and pressure is compressed to 473 mL.
Boyles Law Use Boyles Law to answer the following questions. This law is valid as long as the temperature and the amount of gas are constant. This law describes the relationship between the pressure and the volume of a gas when the temperature.
Quality of your writing 2 pts It is written in complete sentences. This experiment was meant to explore the relationship between pressure and gasses described by Boyles Law. Open the valve and pull the plunger back to set the initial volume of the air in.
APPARATUS Pressure sensor syringe and computer. California State Universit y Los Angeles. Note that if T is held constant throughout the experiment then the ideal gas law reduces to Boyles law.
The gas we use will be air and it will be confined in a syringe connected to a pressure sensor see Figure 1. The purpose of this experiment is to synthesize NaHCO3 by the Solvay process and then convert it to Na2CO3 to carry out an analysis of both compounds by acid-base titration. Boyles Law is identified through the equation PVk.
Tina Jones Heent Interview Completed Shadow Health 1. 2 pts It summarizes the experiment and the result and puts the results in context. Boyles Law states that the pressure P of a gas is inversely proportional to the volume V.
The overall aim of this experiment is to investigate the effect of Boyles Law This is the effect of pressure on volume at a constant temperature This is just one example of how this required practical might be tackled Variables Independent variable Mass m kg Dependent variable Volume V m 3 Control variables. Students will investigate Boyles law using syringes gas pressure sensors and labquest digital data collection units. As long as those two.

Lab 10 Boyle S Law Help Docx Boyle S Law Experiment 1 1 2 1 2 3 3 Record The Pairs Of Data For Pressure Atm And Volume Ml Experiment Course Hero

Laboratory Report Guideline 1 Boyle S Charles S Laws
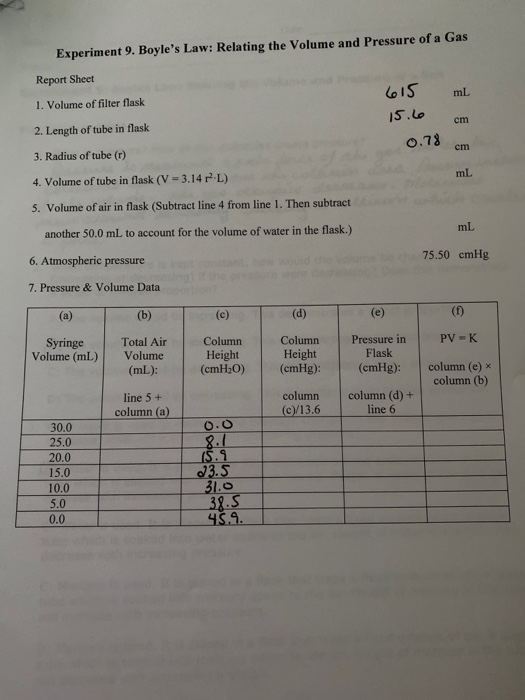
Solved Experiment 9 Boyle S Law Relating The Volume And Chegg Com

Solved Experiment 7 Boyle S Law Laboratory Report Show Any Chegg Com
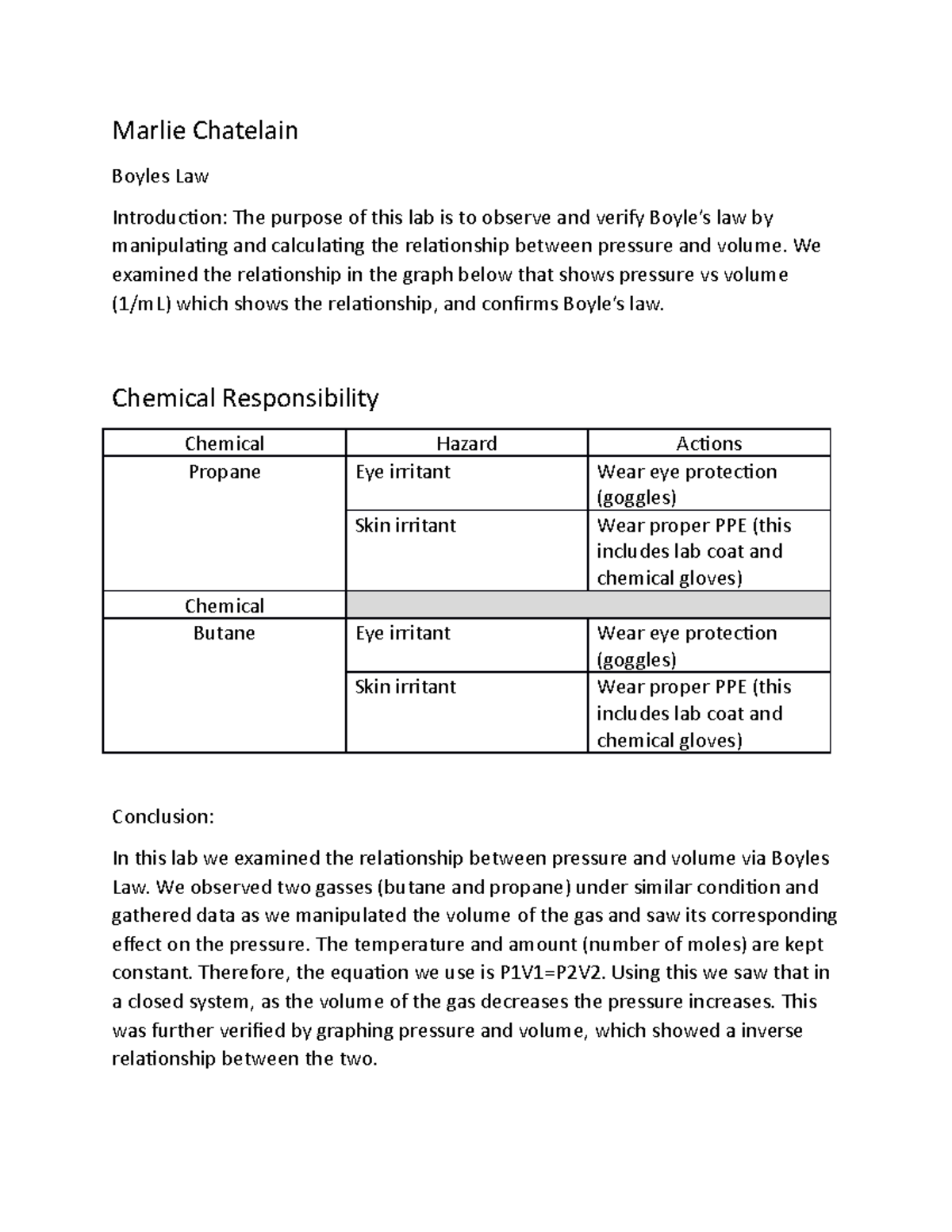
Boyle S Law Lab Chem Lab Marlie Chatelain Boyles Law Introduction The Purpose Of This Lab Is To Studocu

Boyles Law Lab Report Kamaj Stovall 2 4 Boyle S Law Lab Report Purpose Using Boyle S Law Studocu

Ib Ia Gas Law Experiment Testing Boyles Law Charles Law And Ideal Gas Law International Baccalaureate Chemistry Marked By Teachers Com
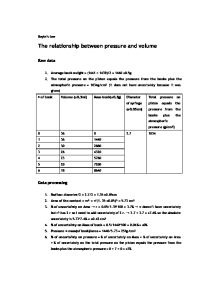
Ib Chemistry Boyle S Law Lab Report International Baccalaureate Chemistry Marked By Teachers Com
Boyle S Law Lab Flinn Scientific

Solved Report For Experiment 18 Instructor Boyle S Law Data Chegg Com

Boyle S Law Report Dcp Ce International Baccalaureate Physics Marked By Teachers Com

Ib Chemistry Charles Law Lab Report International Baccalaureate Chemistry Marked By Teachers Com

Boyle S Law Experiment Chemistrygod